The safe and efficient delivery of oxygen to patients.
Bag valve masks, also called ambu bags (artificial manual breathing units) are used to deliver oxygen to patients who aren’t breathing on their own. But while they have saved many lives, they are not well-suited for treating patients with the COVID-19 coronavirus.
The mask’s main shortcoming is that it must be manually squeezed to force oxygen into the lungs. This works for short periods, but not for long-term care. It can also send varying amounts of oxygen into the lungs and the patient must first be stabilized before given oxygen.
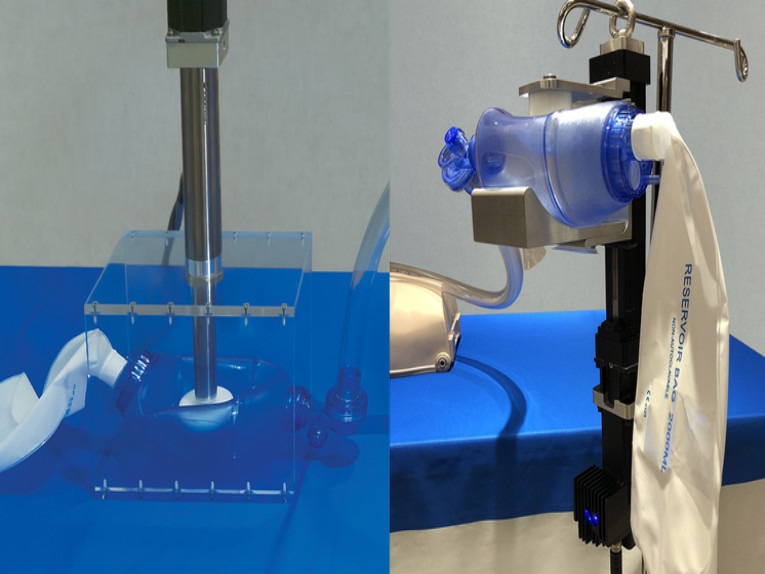
After looking at open-source designs for emergency ventilators, a team of engineers at Tolomatic got together and came up with some new ideas for building ventilators out of ambu bags. Within a week the team had created two designs that use the company’s screw-driven electric actuators. The designs automate the control of air volume and how often it is supplied to patients—a significant improvement over manually operated ambu bags.
Our approach was to automate a traditionally manual process using electric linear actuators and ensure patients would continue to get air for days or weeks. The actuators control the velocity, frequency and stroke length of the move down; how long to pause; and then the velocity, frequency and stroke length of the return move.
The actuators give doctors and nurses complete control over the flow of oxygen going into the patient’s lungs, unlike more traditional camming or pneumatic cylinders we have seen on other open-source designs. Whether the patient is young, old, large or small, they can adjust how much air and how frequently it is delivered to the patient.
We use either an electric rod actuator or a rodless electric actuator. Both are screw-driven linear actuators that convert rotary power from a servo motor into linear motion. Rod-type actuators have a rod which extends and retracts from the main body of the actuator. Rodless actuators lack a rod. Instead, the travel or stroke is contained within the actuator. For this application, the rod actuator needs a housing to hold the ambu bag and the actuator mounts to the housing. The rodless actuator is more compact and can hold and compress the ambu bag within its rails.
Optimal stroke length for both prototypes is 2.5 in., with a minimum of 25 lb of force at the output shaft. Both are rated for at least 100 lb of force. The Direct-drive (or in-line) motor configuration simplifies the design and cuts costs. Add gearing to the design would let it accommodate a wider variety of motors.
These new designs seem to be an improvement over ambu bags modifications that automated the process using single-direction camming or a pneumatic actuator. These do not let healthcare workers adjust the stroke length, only the breath frequency. As a result, the same volume of volume of is delivered to all patients and the resulting motion profile is not like a typical breathing cycle.
Linear actuators, on the other hand, can quickly accelerate and maintain speed over a specified distance, providing more continuous volumes of air per compression and a more typical breathing cycle. The total control of motion from a linear actuator also lets air volumes be adjusted for a patient’s age, size or current needs.
The designs offers reliable long-term performance and will accommodate the addition of alarms in case of motor faults and sensors to monitor airflow quantity and quality.
We are not a medical device manufacturer, and this design has not been approved by any regulatory bodies. However, these concepts limit the patient contact to that of the already approved ambu bag. Our intent is to reach out to other partners, spark some interest and discuss how we can collaborate on an approved design that will help medical personnel trying to save lives.
To date, we have talked with several companies pursuing other concept designs and are interested in the added benefits electric actuation would provide. Our company is ready to offer our motion control expertise to help solve these critical challenges. Companies should contact us for assistance with their applications.
Post time: Mar-29-2021